measuring the thickness of aluminum foil lab|volume of aluminum foil : wholesaling Fold the foil up into a small square and measure its mass using the electronic balance in the weigh room. When finished, return the foil and ruler to the front bench. Analysis: Use these measurements along with the density . Resultado da 16 de out. de 2014 · 他先后多次通过网银向bet365体育网汇款参与博彩,共计40万元。后来,该网站关闭,钱也不知所踪。 赵先生认为,他基于对百度的信任,并通过百度找到的bet365体育网,“百度应该对自己的损失承担责任”。
{plog:ftitle_list}
WEBAnix offers a freemium anime streaming experience with premium features, allowing you to watch anime online for free without registration. A comprehensive collection of anime encompassing a broad spectrum of genres. HD definition for .
volume of aluminum foil
1. Using the procedure you developed in the prelab section, determine the thickness of your sample of aluminum foil in atoms. 2. Adapt your aluminum foil procedure to determine the diameter (also in atoms) of a length of copper wire. Assume the wire is a perfect cylinder. Fold the foil up into a small square and measure its mass using the electronic balance in the weigh room. When finished, return the foil and ruler to the front bench. Analysis: Use these measurements along with the density .
In this lab, you will use the same method that manufacturers use to determine the thickness of aluminum foil. You will also calculate how many atoms thick a piece of foil is.
polarimeter results
Cut out three squares of aluminum foil with sides of the following lengths: 50 mm, 100 mm, and 200 mm. To determine the area of the 50-mm foil square, measure the length of one of its .Ordinary laboratory tools are not suitable for the direct measurement of the thickness of a piece of aluminum foil. Often known properties (such as density) are used to indirectly .The formulas that will enable you to find the thickness of the foil are familiar to you: The volume of a regular object is found by using the formula: where L = length, W = width, and H = height. .From these tools, you are to compute the thickness of a sample of aluminum foil as accurately and precisely as possible. Your lab report should contain a detailed procedure of what you did, .
Akul Murthy In this lab, you will measure mass, area, and volume in order to calculate the thickness of a single sheet of aluminum foil. Procedure 1. Using the meter stick, . This is the video recording of the Aluminum Foil Thickness Full Lab for Big Idea 2 - Atoms in Chemistry 11. Since the lab is relatively long, it is recorded.
Akul Murthy In this lab, you will measure mass, area, and volume in order to calculate the thickness of a single sheet of aluminum foil. Procedure 1. Using the meter stick, cut out a square of aluminum that measures 10 cm on each side. 2. Calculate the area of the square in square meters (area = length × width).The density of aluminum is 2.70 g/cm3. 3. Since the purpose of this activity is to find the thickness of the aluminum foil, and V=L x W x T and A=L x W; these equations can be substituted to give: T = V/A. OBJECTIVES: 1) Correctly apply the principles of significant figures in calculating the thickness of aluminum foil.
Physical Science Lab Manual Chapter 1 Consumer Lab 281 Determining the Thickness of Aluminum Foil Many products such as aluminum foil are too thin to measure easily. However, it is important for manufacturers to know how thick these products are. They wouldn’t be useful if they were made too thick or too thin. Materials: Meter stick Aluminum foil Micrometer Scissors Triple beam balance or electronic scale Graduated cylinder Water Scientific calculator In this lab, you will measure mass, area, and volume in order to calculate the thickness of a single sheet of aluminum foil. Procedure 1. Using the meter stick, cut out a square of aluminum that measures 10 cm on .Lab 0.4: Density and Thickness of Aluminum Foil Student will be able to: • Correctly use measuring instruments with accuracy • Correctly apply the principles of sig nificant figures in measurements and calculations • Correctly use scientific notation in expressing the results of the calculations . Objectives of the Experiment•Calculate the thickness of a piece of aluminum foil •Describe measurements using the appropriate number of significant figures and units •Properly read measuring devices for length, volume, and mass. Materials your students will need: •100mL graduated cylinder •Square foil •Aluminum pellets •Ruler •50mL beaker •Balance
In order to indirectly measure the thickness or "thinness" of a piece of aluminum foil, you have to do a series of calculations. It is determined through in.From these tools, you are to compute the thickness of a sample of aluminum foil as accurately and precisely as possible. Your lab report should contain a detailed procedure of what you did, data tables containing your data, and your calculations should clearly detail how you calculated the thickness from your data. You will be graded both on the
and measure. Purpose: To relate the size of an aluminum atom to the thickness of a piece of aluminum foil by: 1) determine the density of aluminum 2) indirectly measure the thickness of aluminum foil, and 3) convert units using dimensional analysis. Materials: 25 to 100-mL graduated cylinder scissors water aluminum cylinder metric ruler .
The purpose of this lab is to determine the thickness of aluminum foil using the data collected by measuring the aluminum foil. The goal is to measure the length(cm), width(cm), height(cm), thickness(cm), and volume(cm )of the aluminum foil by 3 measuring the length, width, and height with ruler, mass with electronic balance, and using the .
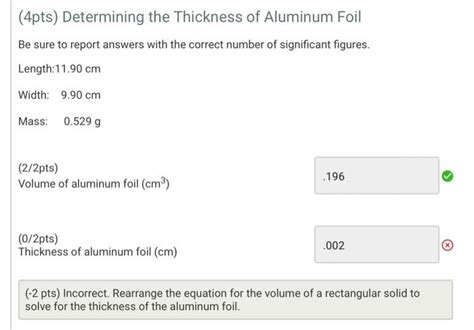
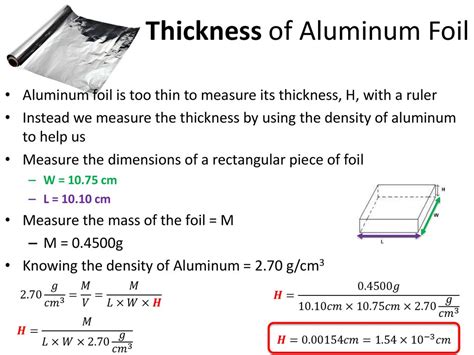
Given the density of the aluminum, 2.70 g/cm³, calculate the thickness of the foil First find the volume using density as a unit factor 0.465g x 1cm³/2.70g=.172cm³ The thickness is found after dividing the volume by its length and width .172cm³/(10.10cm)(10.05cm)=.00170cm Common gauges for aluminum foil range from 6 to 24 microns, with the most commonly used thickness for household foil being 10 to 18 microns. Alloy: The alloy of aluminum foil refers to the specific combination of aluminum and other elements used in .sample paper sample aluminum thickness lab report determination of the thickness of aluminum foil name chemistry, period date exp performed report date. Skip to document. University; High School. . performed Report date Introduction Aluminum foil is a common household material that is so thin it is impossible to measure directly using a ruler .This is the video recording of the Aluminum Foil Thickness Full Lab for Big Idea 2 - Atoms in Chemistry 11. Since the lab is relatively long, it is recorded.
Sample Aluminum Thickness Lab Report Determination of the Thickness of Aluminum Foil Name Chemistry, Period x Date Exp performed Report date Introduction Aluminum foil is a common household material that is so thin it .The Thickness of Aluminum Foil. Take a rectangular piece of aluminum foil and ruler. Use the ruler to measure the length and width of the piece of foil. Fold the foil up into a small square and measure its mass using the electronic balance; Analysis: Use these measurements along with the density of aluminum to calculate the thickness of the foil.This lab experiment aims to determine the thickness of aluminum foil using measurements of foil squares of different lengths. Students will measure the length, area, mass and volume of foil squares to calculate thickness. They .
Al Thickness Lab thickness of aluminum foil lab equations used: measurements used density mass length width height volume 2.6989 4,893g 41.4cm 30.4cm .00144cm. Skip to document. University; High School. Books; Discovery. . for measuring a volume, it would not make a difference. 5.) If the aluminum foil is not pure aluminum (it has several .Name: Jaden Condon How Thick Is Aluminum Foil? Materials: Meter stick Aluminum foil Micrometer Scissors Triple beam balance or electronic scale Graduated cylinder Water Scientific calculator In this lab, you will measure mass, area, and volume in order to calculate the thickness of a single sheet of aluminum foil. Procedure 1.
The Thickness Of Aluminum Foil Calculator is a tool that helps you determine the thickness of an aluminum foil sheet in microns. This calculation can be useful in various scenarios, from ensuring proper heat distribution while . The length and width of the medium piece of aluminum foil is 12.2 cm x 11.8 cm. The mass of the medium piece is 0.87 g.
Lab 2: Density and Thickness of Aluminum Foil Name: Group: Minutes: Grade: Objective:_____ _____ Introduction: Density is the measurement of the amount of matter (mass) in a certain amount of space (volume). . Measurement Value Measurement Value a) Calculate the thickness of the piece of aluminum foil. Show your work below: formula, numbers . Chemistry document from White Rock Christian Academy, 5 pages, Data Analysis Table 1: Data for Aluminum Foil Sheets Sheet Number Type (heavy or normal duty) Length (cm) Width (cm) Mass (g) 1 Normal 24.21 11.29 1.28 2 Normal 24.18 18.3 1.83 3 Heavy 23.96 13.19 1.96 4 Heavy 32.52 23.78 4.43 The graph table presents mea
The Thickness of Aluminum Foil. Now obtain a rectangular piece of aluminum foil. Use the ruler to measure the length and width of the piece of foil. Measure the mass of the foil using the electronic balance. Analysis: Use these measurements along with the density of aluminum to calculate the thickness of the foil.
In this lab, we test on the common household item Aluminum foil. Aluminum foil is extremely thin and is impossible to determine the thickness of said Aluminum foil with a simple ruler. Density is what makes that possible, if we use Mass divided by volume to the well-known density of Aluminum Foil of 2.70 g/cm^3. 1.4.3 Lab: Measuring and Estimating Wet Lab Physics Name: Ashlynn Smith Date: 09/29/21 How Thick Is Aluminum Foil? Materials: Meter stick Aluminum foil Micrometer Scissors Triple beam balance or electronic scale Graduated cylinder Water Scientific calculator In this lab, you will measure mass, area, and volume in order to calculate the thickness of a .This document provides instructions for a lab experiment to determine the thickness of a piece of aluminum foil. Students are asked to design a procedure to measure the thickness, record observations of the aluminum foil's physical properties, and answer analysis questions about the experiment. The questions address the accuracy and precision of the data collection, sources .
polarimeter rotation measurement
web20 de jan. de 2019 · 参考图片神器《PureRef》操作全翻译. 画画,建模,设计,辣么多参考图片,可是却木得两块屏幕。. 怎么办?. 快来试试《PureRef》吧。. 23333. 做这个专栏的起因是向同事朋友安利这个神器的时候,发生了件很蛋疼的事情,就是他木得中文也没有教 .
measuring the thickness of aluminum foil lab|volume of aluminum foil